HEALTH CARE : Cardiac Sciences Wins FDA OK to Test Device to Stem Heart Attacks
- Share via
It was a leap of faith when Howard Cooper left Trimedyne Corp. last year to start his own company and begin designing a medical device that would automatically stem the onset of heart attacks.
His plan is one step closer to fruition.
The Food and Drug Administration has given his company, Cardiac Sciences Inc. of Irvine, the green light to test a one-of-a-kind, non-implantable heartbeat regulator on about 300 patients.
Human clinical testing is required before the FDA issues “premarket approval,” which is the final phase before a product is allowed to be sold to the public. The FDA reviews design and clinical trial documents during the premarketing approval process.
If given final FDA approval, Cardiac Sciences could tap into a multimillion-dollar business, helping the estimated 1.5 million people in the United States who suffer heart attacks each year.
“There are a lot of people who are just ticking time bombs out there,” Cooper said.
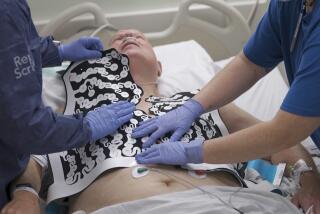
Cooper’s bedside automatic cardioverter defibrillator uses sensors to detect a patient’s irregular heartbeat and send a controlled jolt of electricity to the patient’s heart, quelling the type of heart attack that leads to sudden cardiac death.
Eventually, Cooper said, the automatic defibrillator will be designed small enough to allow patients to walk around, even outdoors.
Testing of the device will begin by the end of the year at UCI Medical Center in Orange.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.